A cleanroom or clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research including the manufacture of pharmaceutical items integrated circuits crt lcd oled and microled displays.
Clean room environment ppt.
Phen602 pharmaceutical facility design fall 2008.
Clean rooms and controlled environments iso vs fs209e.
In biotechnology and medicine cleanrooms are used when it is necessary to ensure an environment free of bacteria viruses or other pathogens.
Four fundamental rules apply to cleanrooms.
Pic s general paragraphs cleanrooms slide 18 of 68 november 2014 tehran university of medical sciences school of pharmacy clean areas.
Clean rooms need a lot of air and usually at a controlled temperature and humidity.
Clean areas for carrying out less critical stages in the manufacture of sterile products.
Many companies claim to provide expert training but iest s accredited expertise as an international standards developer assures confidence in the effectiveness of the iest learning path certificate program for you and your personnel.
Typically used in manufacturing or scientific research a cleanroom is a controlled environment that has a low level of pollutants such as dust airborne microbes aerosol particles and chemical vapors.
Managing risk to cleanroom products and processes begins with trained personnel at all levels.
A clean rooma clean room is a rigorously controlled environment that has a low level of environmentalis a rigorously controlled environment that has a low level of environmental pollutants such as dust airborne microbes aerosol particles and chemical vapors.
A controlled environment such as a clean zone or clean room is defined by certification according to a relevant clean room operational standard.
This means that in most facilities the cleanrooms air handling units ahu consume over 60 of all the site power.
26 clean room training copyright jhu apl 2002 all rights reserved homewood key contact information.
Key differences iso generally requires fewer sampling locations than fs 209e with iso number of sample locations is based on clean room area whereas with fs 209e it is based.
Entry through airlocks for personnel and or for equipment and materials.
Grade c and d.
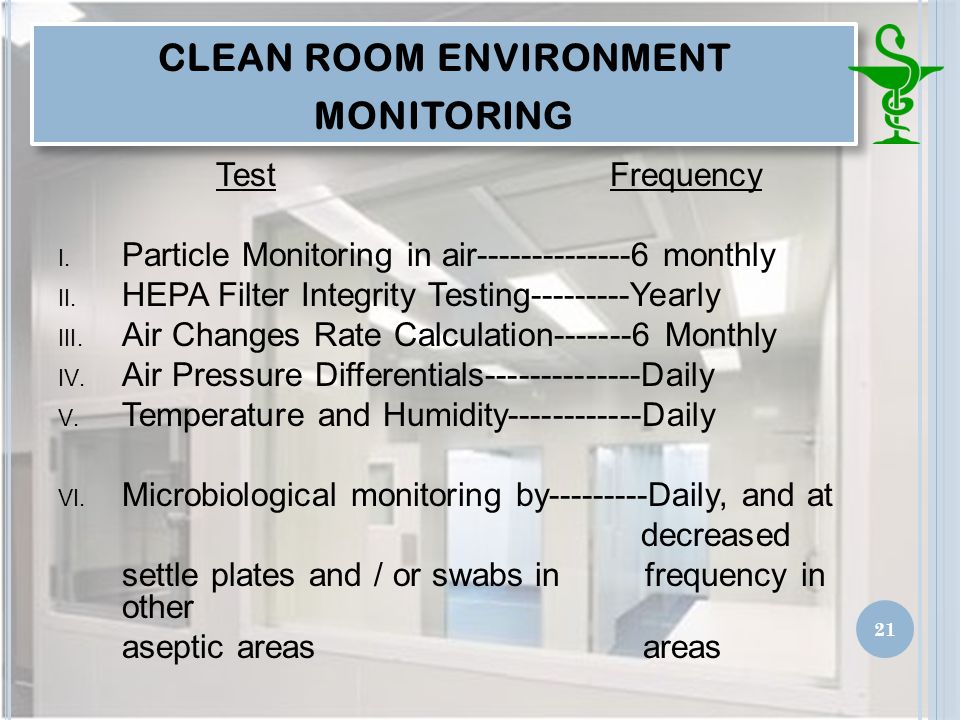
Parameters that are evaluated include filter integrity air velocity air patterns air changes and pressure differentials.
1 first contaminants must not be introduced into the controlled environment from the outside.